White Board Challenge
White Board Winner Announced
Congratulations to the team behind the cutting-edge presentation
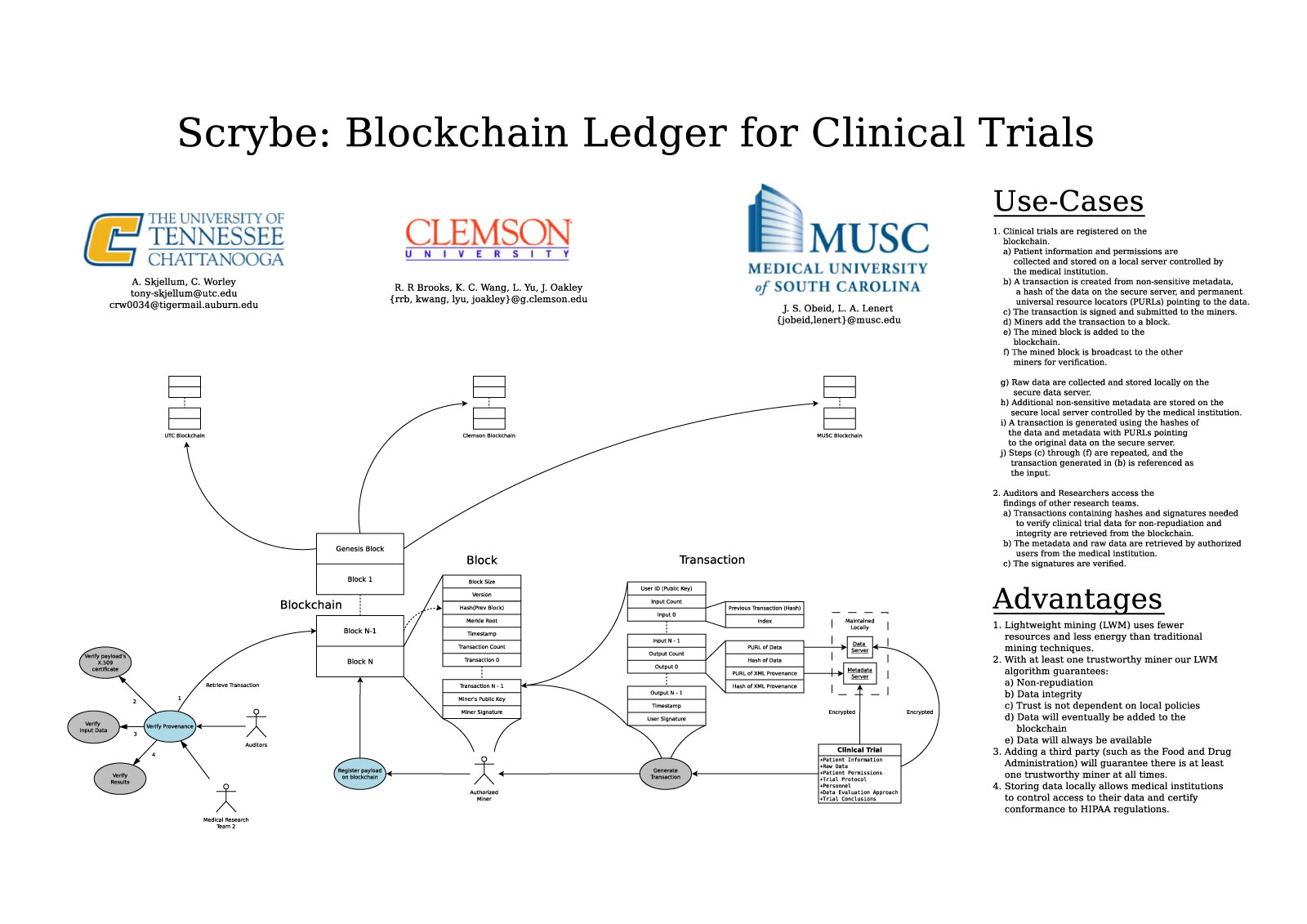
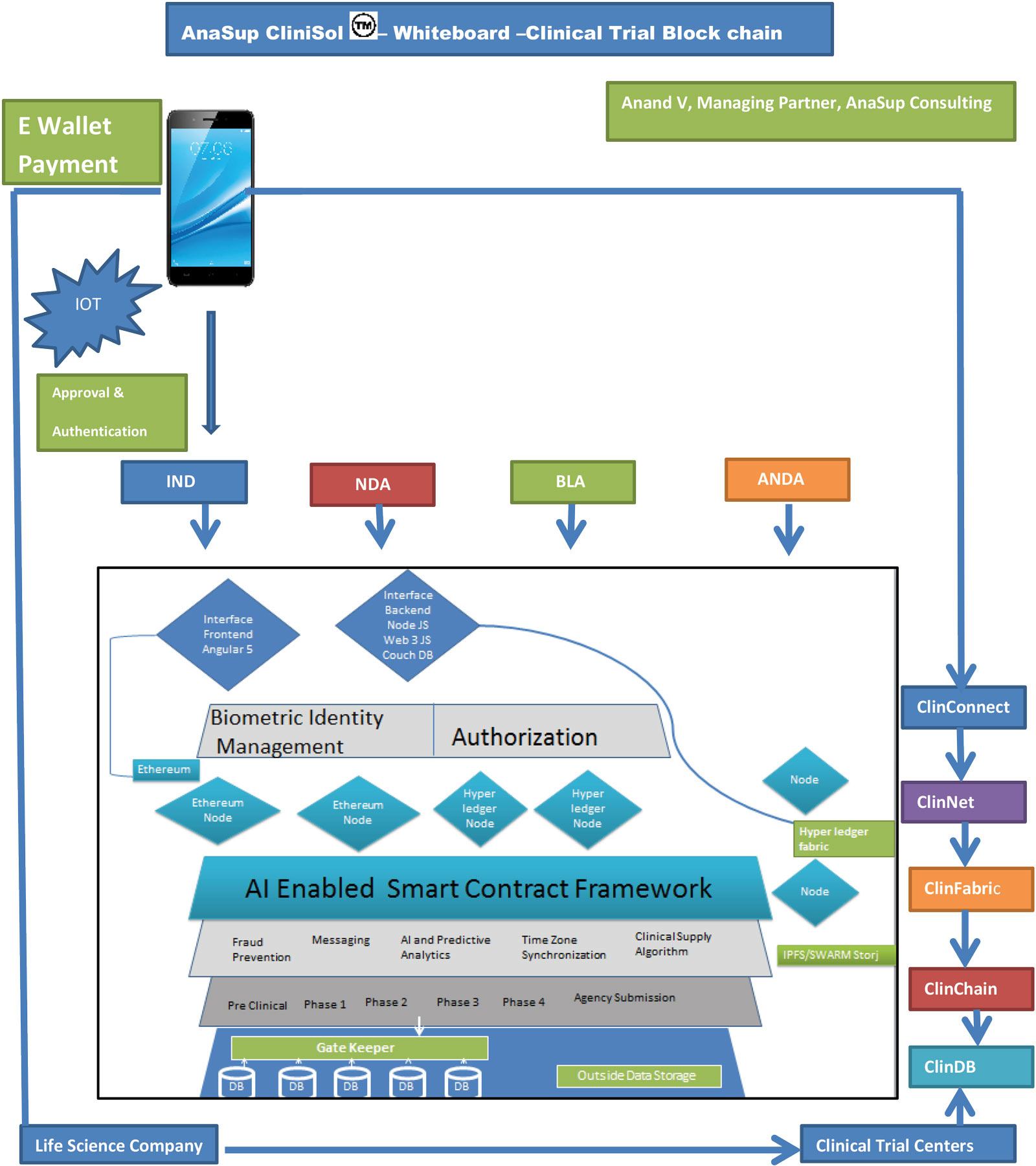
Scrybe: A Blockchain Ledger for Clinical Trials
About the IEEE Blockchain for Clinical Trials White Board Challenge
We tasked progressive and emerging technologists to present a blockchain/DLT (distributed ledger technology) reference model for clinical trials application. The reference model must include either a private, public or hybrid application using a distributed ledger technology and/or smart contracts.
The Challengers were to demonstrate:
- Thorough understanding of the problem the blockchain/DLT will solve
- Understanding of the dynamics around the users of the technology
- Present a detail record of how the users would use the technology
- Sketch the reference model design
Special Thanks to All the Entrants. Your Submissions Inspired our Audience
Please note the authors of the reference designs listed here are those who will be in attendance to discuss their work. There additional co-authors to these various projects and are noted on the white papers and synopsis on the event website.
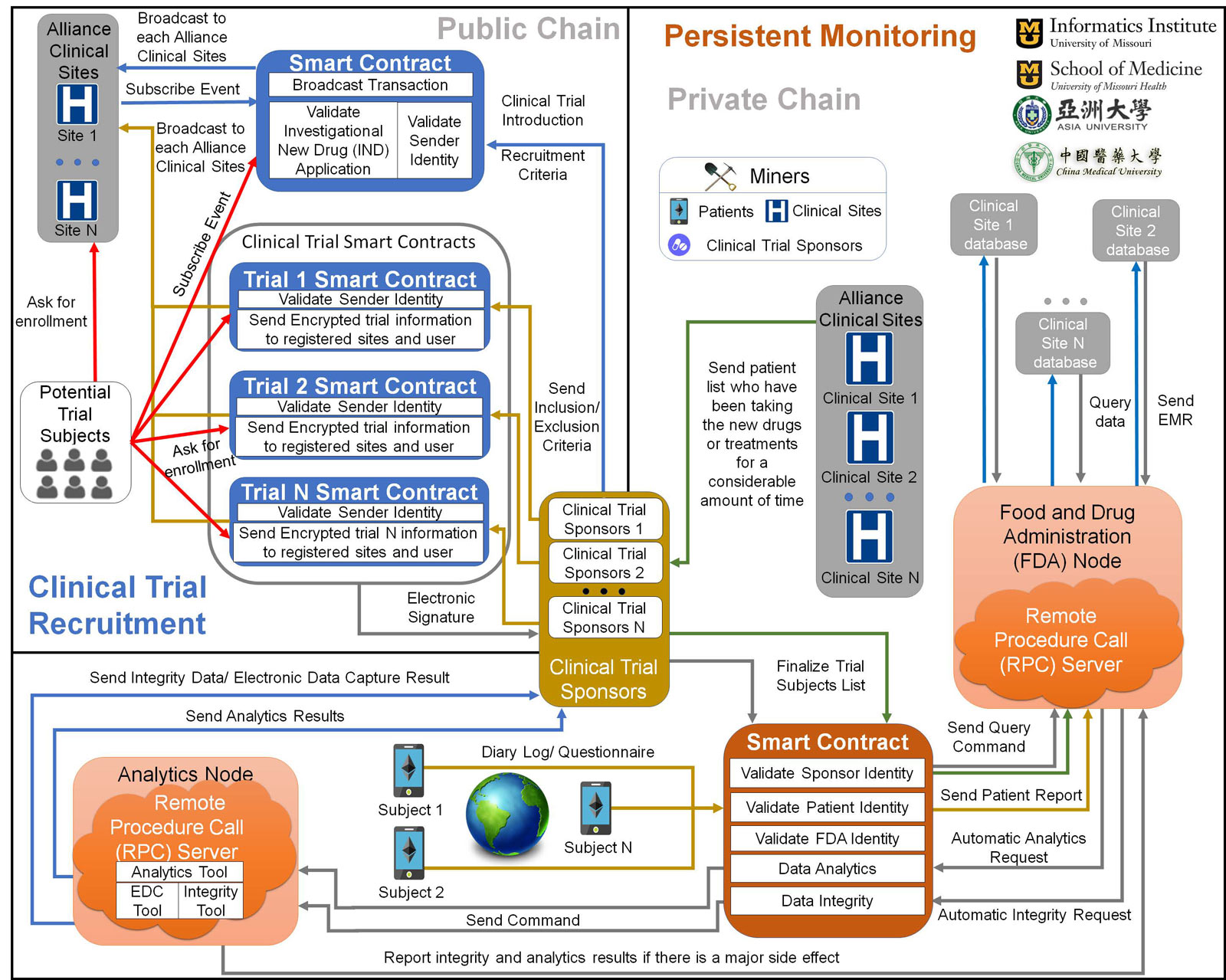
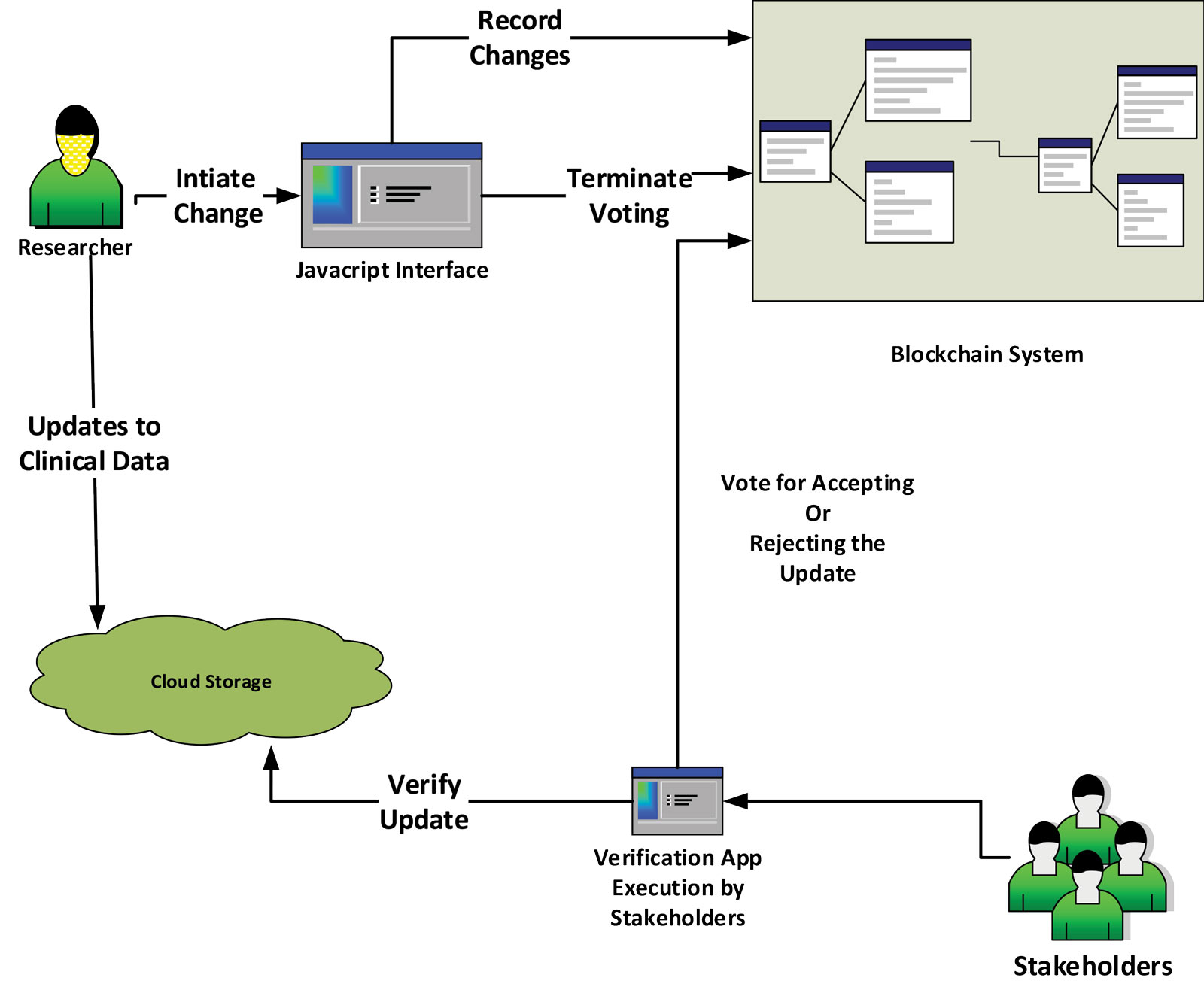
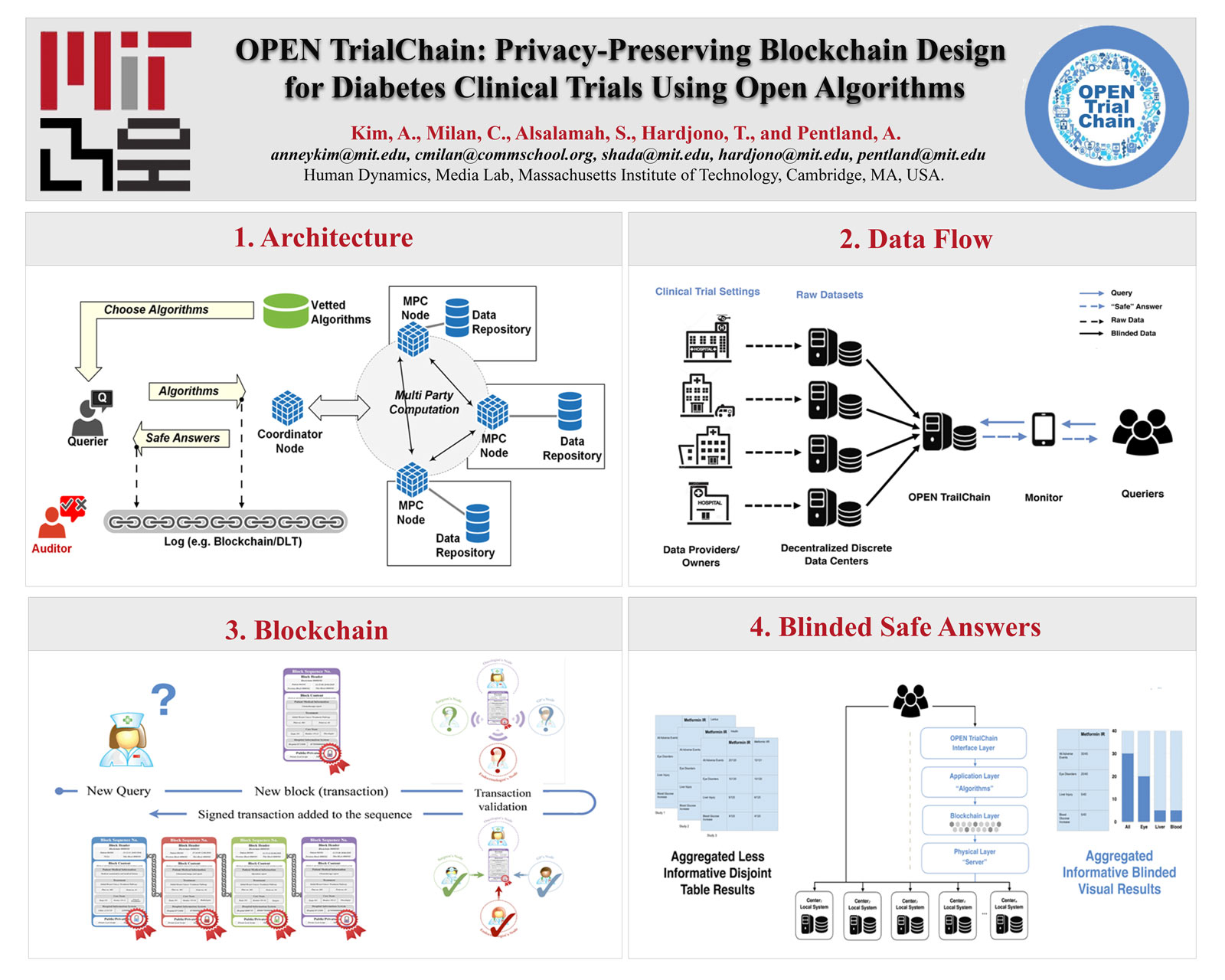
A Hybrid Blockchain Design for Patient Recruitment and Persistent Monitoring for Clinical Trials
Chi-Ren Shyu
University of Missouri & Asia University
Please visit the Attendee Prep page to view the full presentations.
Inquiries
Please contact Maria Palombini, m.palombini@ieee.org.